CHELATION OF METAL CATIONS WITH CURCUMINOIDS IN TREATMENT OF ALZHEIMER’S DISEASE
Introduction
Curcuminoids are potent antioxidants. They are mostly comprised of three main chemical compounds – curcumin, demethoxycurcumin, and bisdemethoxycurcumin. Curcumin is the main component, and has the appearance of orange crystalline powder and it is stable and incompatible with strong oxidizing agents [3]. Its compound source is from the curcuma zedoraria, or turmeric, which is commonly found in Southeast Asia and in India, and is a member of the ginger family [4].
Figure 1: Turmeric plant with rhizomes (orange offshoots at bottom)
Curcumin has been said to be an anti-inflammatory and anticancer agent, helping to ease those with Alzheimer’s and other diseases, despite its low efficacy in several disease models [4]. It is a powerful antioxidant and it raises the level of antioxidant enzymes and scavenges reactive oxygen species and can protect the body from them [3]. It is believed that it helps to ease Alzheimer’s symptoms because it reduces the chronic inflammation of affected nerve cells that cause dementia and loss of thinking and judgement. It appears that the gradual loss of brain function characteristic for Alzheimer’s disease is connected to two main forms of nerve damage: the development of β-sheet-rich fibrils inside the neurons and β-amyloid plaques outside of the neurons.
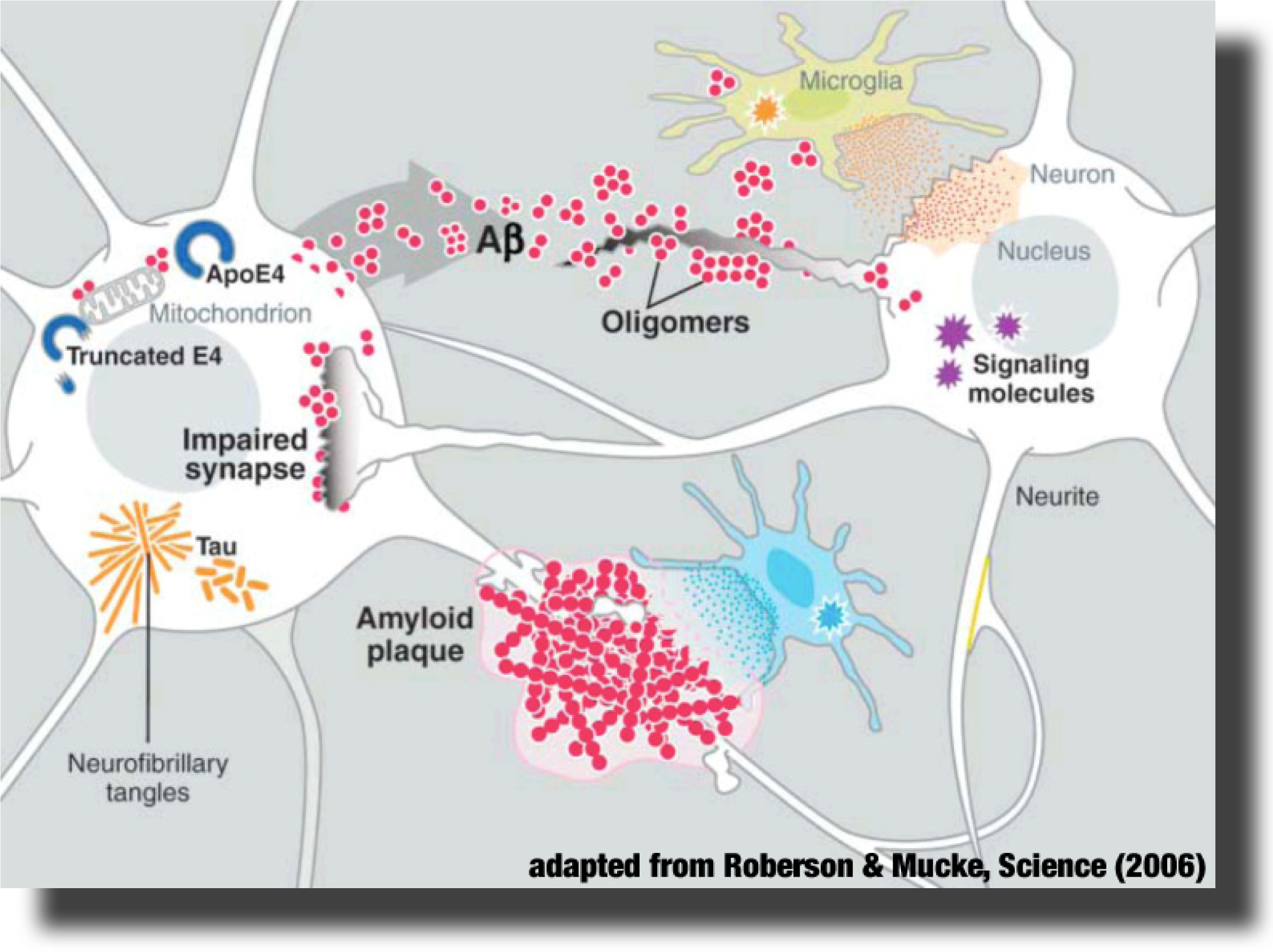
Figure 2: β-sheet-rich fibrils in the form of tangles in a neuron and the β-amyloid plaque inhibiting signaling to another neuron
The fibrils are insoluble twisted fibers and the plaques are clumps of cellular material and fragments of the amyloid-β-peptide [8]. The degradation can be induced by many factors, including the presence of excessive metal ions such as aluminum, iron, and zinc. Due to its lipophilic (lipid dissolving) nature, curcumin can cross the blood–brain barrier, destabilize the preformation of β-amyloid fibrils, and eventually bind to plaques and inhibit formation of β-amyloid fibrils, while also chelating the metal ions, which inhibits damage to the brain [8].
Demethoxycurcumin and bisdemethoxycurcumin are derivatives of the curcumin and have similar properties and are used as inhibitors, as well as curcumin itself, but curcumin seems to be the focus in all the articles and studies that have been performed.
Curcumin is a PAINS, or a pan-assay interference compound, which is a compound that shows activity in multiple types of assays. Assays are a testing of metals, and PAINS show these assays by interfering with the assay readout, rather than through specific compound interactions. Compounds that exhibit PAINS-type behaviors demonstrate: metal chelation, redox reactivity, covalent bonding of proteins, etc. [5].
Figure 3: Some well-known classes of pan assay interference compounds (PAINS)
Figure 4: Demonstration of PAIN interfering with assay breakout, rather than specific interaction
Chemistry of Curcuminoids
Below are the structures of the three major types of curcuminoids:
They all resemble each other because of the two phenyl rings and hydroxyl groups in their structures, and because of the various hydrocarbon chains (keto groups).
They have slight variations however, because of the different functional groups they possess. These variations allow some of the curcuminoids to have better and different tendencies and reactivity than others. Curcumin has several different functional groups such as methoxy groups, and unsaturated carbonyl groups. The demethoxycurcumin has one less methoxy group than in curcumin. Lastly, bisdemethoxycurcumin has no methoxy groups, which causes the lesser number of atoms as seen in the molecular formula, and has two hydroxyl groups, similar to curcumin. This difference in structure of the curcuminoids may be able to account for why curcumin is more effective against Alzheimer’s disease.
Figure 5: Curcumin (Diferruloylmethane)
Molecular formula: C21H20O6
It is the most active ingredient in turmeric. The three curcuminoids make up 2-6% of the rhizome content (rhizomes in figure 1), but approximately 80% of that percentage is made of curcumin [3].
Figure 6: Demethoxycurcumin
Molecular formula: C20H18O5
Figure 7: Bisdemethoxycurcumin
Molecular formula: C19H16O4
It has the least amount of atoms in its structure, but it is the most resistant to alkaline degradation.
Separation/purification of Curcuminoids
Since it is known what percentage of each curcuminoid makes up the faction (curcumin being the most abundant), there are ways to separate the substances from each other using crystallization. After crystallization occurs, chromatographic treatment of the remaining liquid must occur to obtain the pure demethoxycurcumin to increase the yield of pure curcumin [11]. In the chromatographic process, a mixture of chloroform and methanol is used as the mobile phase, and silica gel is used as the stationary phase. Each fraction isolated in the process was characterized by high-performance liquid chromatography (HPLC) and mass spectrometry (LC-MS) techniques, and the resulting forms are analyzed. Overall, the combined purification method recovered from the crude curcumin: 88.5% curcumin, 79.7% demethoxycurcumin, and 68.8% of bisdemethoxycurcumin, in highly pure form curcumin (100%), demethoxycurcumin (98.6%), and bisdemethoxycurcumin (98.3%) [11].
Synthetic Method to Synthesize Curcumin
If there is no way to purify or separate curcuminoids in a certain environment, there is also a method to synthesize curcumin.
Curcumin synthesis can be performed when Acetyl acetone and boric anhydride (catalyst) are dissolved in ethyl acetate and vanillin is added. The reaction mixture is stirred at 80 °C for 30 minutes and then n-butylamine can be added dropwise over 30 minutes into the ethyl acetate and the mixture stirred for 4 hours at 80 °C. Upon complete consumption of the starting material, hydrochloric acid can be added and the mixture should be stirred. Organic layers of water-immiscible solvents are separated and extracted with an appropriate solvent. The combined organic layer is then washed with water and dried over sodium sulfate, filtered, and concentrated under reduced pressure. The crude product is recrystallized from methanol to give curcumin as a yellow solid [12].
Figure 8: Acetyl acetone and vanillin bind to create curcumin (synthesis)
Method of Action: Chelation
Chelation is the type of bonding of ions and molecules to metal ions. The formation of bonds occurs between two or more separate binding sites within the same ligand and a central atom [6]. A ligand is an ion or molecule (functional group) that is bonded to a metal atom. It is an electron donor since metals are electron acceptors, and the ligands donate pair(s) of electrons to form the bonds to metals. Water is a common ligand in many biological and chemical interactions [7].
Curcumin reacts with metals such as aluminum and zinc ions, because curcumin is a metal ion chelator, specifically iron. As an iron chelator, it represses ferritin translation, according to an iron chelation study, thus causing the levels of ferritin protein to reduce [2]. A study done about the metal chelation therapy in Alzheimer’s disease illustrates that curcumin significantly reduced the toxic metals found inside the neurological cells of the hippocampus in the brain, which affects Alzheimer’s patients and causes them to lose their long-term memory specifically [1]. The curcumin can help to alleviate these toxins in the brain, because when metal ions seem to abnormally accumulate in the brain with these neurodegenerative diseases and aging, curcumin is able to bind toxic metals and form tight and inactive complexes, which also renders the metals inactive [1]. Curcumin also can bind with redox-active metals (such as the iron and copper) and creative curcumin-metal complexes. These complexes can be formed because of the hydroxyl and carbonyl groups that make up the structure of the curcuminoids.
Figure 9: Curcumin bonds with metal because of metal complexes that allow for chelation and inhibiting of harmful metals
Evidence has shown that curcumin can inhibit inflammatory cell proliferation (rapid increase of inflammatory cells), invasion, and angiogenesis (development of new blood cells) through the down-regulation (suppressing in response to a stimulus) of inflammatory transcription factors, cytokines, protein kinases, and enzymes that promote inflammation [13]. This occurs through phosphorylation of signal transducer since the phosphate groups is needed to activate relay proteins in the neurons.
Neuron Firing/Synopsis
Neuron firing and synopsis are typically signals that occur in neuron cells in the human brain. The neuron cells are used to detect outside environment, internal environment of an organism, to adapt and show behavior responses to signals, and to control the body based on the responses. Alzheimer’s patients can be seen to struggle with this because of the degradation of these cells [9]. On one side of neurons is dendrites, which are branching structures that receive the signals from the axons of other neurons. Axons contain synapses that usually connect with the dendrites or even directly to muscles in the body [9]. The synapses are where the neuron ends, and it releases neurotransmitters into the synaptic cleft of another neuron, also known as neuron firing. Neuron firing can occur because of the action potential, or electric pulse, that travels down the axons to the synapses. This occurs when part of the membrane allows positively charged ions to open a protein channel (such as potassium or sodium), while negatively charged ions leave (such as chloride). This causes a rapid increase in positive charge of the nerve fiber, and allows it to move down a chain of neurons, like a wave [10]. Alzheimer’s disease disrupts the way the electrical charges travel with neurons, and the activity of neurotransmitters, thus causing the symptoms of dementia and other disabilities.
Figure 10: Neurotransmitters released during a nerve impulse from synapse
References
Figure 7: Bisdemethoxycurcumin. (n.d.). Retrieved from Chem Spider database.
1) Challenges Associated with Metal Chelation Therapy in Alzheimer's Disease. (n.d.). National Center for Biotechnology Information. https://doi.org/10.3233/JAD-2009-1068
Figure 5: Curcumin. (n.d.). Retrieved from Chem Spider database.
Figure 6: Demethoxycurcumin. (n.d.). Retrieved from Chem Spider database.
2) Iron chelation in the biological activity of curcumin. (n.d.). National Center for Biotechnology Information. https://doi.org/10.1016/j.freeradbiomed.2005.11.003
3) Is Curcumin Different From Curcuminoids? (n.d.). Retrieved from Turmeric for Health website: https://www.turmericforhealth.com/turmeric-queries/is-curcumin-different-from-curcuminoids
4) Mishra, S., & Palanivelu, K. (n.d.). The effect of curcumin (turmeric) on Alzheimer's disease: An overview. National Center for Biotechnology Information. https://doi.org/10.4103/0972-2327.40220
5) Nelson, K. M., Dahlin, J. L., Bisson, J., Graham, J., Pauli, G. F., & Walters, M. A. (n.d.). The Essential Medicinal Chemistry of Curcumin: Miniperspective. Journal of Medicinal Chemistry, 1620-1637. Retrieved from https://pubs.acs.org/doi/pdf/10.1021/acs.jmedchem.6b00975
6) Chelation. (n.d.). Gold Book. https://doi.org/10.1351/goldbook.C01012
7) Definition of ligand. (n.d.). Retrieved from Chemicool.com website: https://www.chemicool.com/definition/ligand.html
Figure 3: PAINS: Pan-assay interference compounds [Photograph]. (n.d.). Retrieved from http://slideplayer.com/slide/5275189/17/images/4/PAINS:+Pan-assay+interference+compounds.jpg
Figure 4: PAINS figure [Photograph]. (n.d.). Retrieved from https://upload.wikimedia.org/wikipedia/commons/thumb/3/34/PAINS_Figure.tif/lossy-page1-330px-PAINS_Figure.tif.jpg
8) Wanninger, S., Lorenz, V., Subhan, A., & Edelmann, F. T. (n.d.). Metal complexes of curcumin – synthetic strategies, structures and medicinal applications. Royal Society of Chemistry. https://doi.org/10.1039/C5CS00088B
9) Neuron basics. (n.d.). Retrieved from http://animatlab.com/Help/Documentation/Neural-Network-Editor/Neural-Simulation-Plug-ins/Firing-Rate-Neural-Plug-in/Neuron-Basics
10) What is an action potential and how do neurons fire? (n.d.). Retrieved from https://www.verywellmind.com/what-is-an-action-potential-2794811
Figure 8: Synthesis of curcumin by condensation of acetyl acetone and vanillin [Photograph]. (n.d.). Retrieved from https://www.researchgate.net/profile/Chanin_Nantasenamat/publication/266387551/figure/fig2/AS:392255329325057@1470532366031/Synthesis-of-curcumin-by-condensation-of-acetyl-acetone-and-vanillin.png
Figure 10: Synapses and neurotransmitters [Photograph]. (n.d.). Retrieved from https://www.alz.org/braintour/images/synapses_neurotransmitters.jpg
11) Heffeman, C., Ukrainczyk, M., Gamidi, R. K., Hodnett, K., & Rasmuson, &. C. (n.d.). Extraction and Purification of Curcuminoids from Crude Curcumin by a Combination of Crystallization and Chromatography. Organic Process and Research Development. https://doi.org/10.1021/acs.oprd.6b00347
12) Ahmed, M. (n.d.). Curcumin: Synthesis optimization and in silico interaction with cyclin dependent kinase. HRCAK. https://doi.org/10.1515/acph-2017-0023
Figure 1: Turmeric plant [Photograph]. (n.d.). Retrieved from https://encrypted-tbn0.gstatic.com/images?q=tbn:ANd9GcRlnkNEctYtHYxK2GX0S5aA7R7wf9AUlHzjIKTQVsXWub-HAAvuRA
Figure 2: Amyloids [Photograph]. (n.d.). Retrieved from https://www.cmu.edu/biolphys/smsl/pictures/AD%20basics%20shadow.jpg
Figure 9: Metal complexes designed for curcumin to bond [Photograph]. (n.d.). Retrieved from http://pubs.rsc.org/services/images/RSCpubs.ePlatform.Service.FreeContent.ImageService.svc/ImageService/Articleimage/2014/CS/c4cs00026a/c4cs00026a-f18_hi-res.gif
13) Shehzad, A., Rehman, G., & Lee, Y. S. (n.d.). Review Article - Curcumin in Inflammatory Diseases. In Review Article - Curcumin in Inflammatory Diseases. (Reprinted from International Union of Biochemistry and Molecular Biology, 39, 69-77, 2013)
- Liliana Torres



No comments:
Post a Comment